The nature of scienceTypical sequence of events leading to an established scientific theory:
 so that the results can be duplicated. There is no science without this ability to duplicate other scientist’s observations! If a scientist’s observations are comprehended in terms of analogies with other objects or concepts, this is called a model. The scientist does more experiments to test this model. If the hypothesis and the model fit all of the data from all experiments, and if the scientist can explain these data in terms of other more primitive scientific phenomena, this explanation is called a scientific theory. A model helps to organize a body of data. A theory can be used to predict the results of future experiments. A scientific law is a concise statement or mathematical equation about a basic relationship in nature. so that the results can be duplicated. There is no science without this ability to duplicate other scientist’s observations! If a scientist’s observations are comprehended in terms of analogies with other objects or concepts, this is called a model. The scientist does more experiments to test this model. If the hypothesis and the model fit all of the data from all experiments, and if the scientist can explain these data in terms of other more primitive scientific phenomena, this explanation is called a scientific theory. A model helps to organize a body of data. A theory can be used to predict the results of future experiments. A scientific law is a concise statement or mathematical equation about a basic relationship in nature. |
|
The Big Bang hypothesis (Chapter 2) regarding the sudden expansion of the universe around fourteen billion years ago has now been corroborated by so many experimental observations that it is now an accepted model. Some scientists have reservations about certain parts of the model. It is not a theory because it cannot explain what happened earlier than a fraction of a second during the Big Bang, and it cannot predict with certainty the future fate of the universe. However, it is currently the most popular model available to explain where we are in the evolution of the observable universe. On the other hand, the law of gravity allows everyone to predict exactly the attractive gravitational forces between all objects from tennis balls to planets. There are no scientists who publicly dispute this law. There have been no exceptions to this law! If there were, it would cease to be a scientific law. Exercise 1-1 Models, theories, and laws Can you name some other scientific models, theories, and laws? Justify their designations according to the above definitions. |
|
Chemistry, natural sciences, and medicine
Fields of Chemistry
Traditionally chemistry has been divided into five fields: organic (the chemistry of carbon chains or rings with or without oxygen and nitrogen and other elements-see below for definitions of carbon, oxygen, and nitrogen), inorganic (deals with substances that don’t contain carbon as their principal element), analytical (deals with techniques that yield information about chemical systems), physical (primarily theoretical chemistry), and biochemistry (chemistry of biological systems). Organic 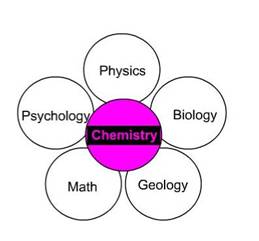 The barriers separating these fields are falling rapidly. Chemists like to designate chemistry as the “central” science (see figure to the right) because all sciences deal in one way or another with chemicals and chemical concepts. There is a great deal of overlap between chemistry biology, physics, and geology. There has been a gradual merging of chemistry and other fields such as molecular biology and biochemistry, geochemistry (geology and chemistry), biogeochemistry (biology, geology, and chemistry) and chemical physics (chemistry and physics). Advances in chemistry have been crucial to nearly all advances in the medical sciences. Some of these advances have depended on new chemical techniques and others have depended on new chemicals synthesized in the laboratory. The barriers separating these fields are falling rapidly. Chemists like to designate chemistry as the “central” science (see figure to the right) because all sciences deal in one way or another with chemicals and chemical concepts. There is a great deal of overlap between chemistry biology, physics, and geology. There has been a gradual merging of chemistry and other fields such as molecular biology and biochemistry, geochemistry (geology and chemistry), biogeochemistry (biology, geology, and chemistry) and chemical physics (chemistry and physics). Advances in chemistry have been crucial to nearly all advances in the medical sciences. Some of these advances have depended on new chemical techniques and others have depended on new chemicals synthesized in the laboratory. |
Fields of Chemistry |
What is chemistry? Matter? Mass? Weight?
Chemistry is the study of the properties, composition, and structure of matter, and the changes matter undergoes. Matter is anything that possesses mass when at rest and is observable by the senses. Mass is a measure of a body’s resistance to being accelerated. Therefore, a truck is more difficult to move than a small car because there is more resistance to accelerating the larger mass of the truck.
|
Definition
of chemistry |
|
Mass |
Example 1-2 Mass and weight
Describe two consequences of increasing the mass of an object. Will these consequences also affect the weight of that object?
Increasing the mass of the object will increase the weight of the object on Earth. It will also require more force to increase the velocity of the object, that is, accelerate the object. If the object is in space it will result in no difference in weight because the weight is still zero, but the mass will increase by the same amount as on Earth. |
The Chemical Building BlocksOne of the building blocks of matter is the atom. An atom is the smallest component of matter
Atoms can be broken down into their component parts. For example, if we heat a hydrogen atom (Fig. 1-1) to temperatures comparable with those on the Sun, we obtain an exceedingly small, exceedingly light, negatively charged (–) particle called an electron and an exceedingly small nucleus consisting of a single, positively charged (+) proton. All atoms have nuclei and all nuclei have at least one proton. The mass of a proton is several thousand times greater than the mass of an electron. |
Atoms contain a single nucleus and electron(s) |
||||||||||||||||||
All atoms consist of a single nucleus and one or more electrons. Atoms other than hydrogen have more than one proton in their nuclei.
|
Atomic number = # protons in nucleus |
Periodic Table
|
Periodic Table |
|
Exercise 1-2 Atomic numbers and elements in the periodic table | ||
|
The symbol H is used to represent a neutral hydrogen atom, which is composed of an intimately bound negative electron and positively charged proton. Each substance containing only atoms with the same atomic number (Z) is designated as an element. Elemental carbon is a substance that is composed of atoms that contain 6 protons in each of the atomic nuclei making up the elemental substance. There may be several different forms of an element called allotropes. For example, there are three forms of elemental carbon: diamond, graphite, and recently discovered forms called “buckyballs” and “carbon nanotubes.” The differences in the external properties among these forms are explained by the different internal spatial arrangements of the carbon atoms. |
Element |
|
| |||||
| Diamond | Graphite | Buckyball (above), at the atomic level, with carbon atoms at the intersections of the lines in the general shape of a miniature soccer ball; discovered in soot. There are 80 C (carbon) atoms in one form of this element. | Allotropes |
||
Superscripts in the upper right hand corner of an element represent the net charge of the chemical species, – for an excess unit of negative charge and + for an excess unit of positive charge. The hydrogen atom, H, containing one proton and one electron, has no superscript because the single positive and the single negative charges cancel each other’s charge and the atom is electrically neutral. The symbols representing the electron and proton are e– and H+, indicating each has a net charge of one, but also indicating that they are oppositely charged. |
Hydrogen Atom |
An ion is an atom or a group of atoms with a net positive or negative charge.Thus a hydrogen atom without its electron is a positive ion (H+), a proton. A high temperature plasma is composed, in part, of positively charged ions and negatively charged electrons. Minutes after the Big Bang, according to the model, a very high temperature plasma consisting of neutrons, positively charged nuclei, and negatively charged electrons is postulated to have formed (Chapter 2). |
Ions and Plasma |
Attraction and repulsion of charged particlesExperimental measurements in the laboratory demonstrate that:
These observations lead to the general principle that, at room temperature, oppositely charged particles attract each other and similarly charged particles repel each other. Thus, when free electrons encountered free electrons, and free protons encountered free protons in the Big Bang plasma, there should have been, at least initially, a strong repulsion. |
Charged Particle Interactions |
However, it also has been experimentally established that when temperatures are exceedingly high, as they must have been in the Big Bang plasma, and particle speeds are very high, positively charged particles in a plasma can undergo forceful enough collisions to overcome like-charge repulsion at very short distances between nuclei. When nuclei get this close, different nuclear forces take over so that attractive nuclear forces are dominant and nuclear reactions are now possible. Because of this, new nuclei and therefore new atoms can form during such high-energy nuclear collisions. |
|
Symbols used in nuclear reactionsIn order to accurately represent nuclear reactions, it is convenient to provide the following information regarding particles in the proposed Big Bang plasma: Mass Number (M) Net charge
1. The chemical identity of the atoms; this(#protons+#neutrons) Symbol of
Atomic number (Z)Element (#protons in nucleusdetermines element!) is represented by one large capital letter representing the symbol of the element (e.g., “H” stands for hydrogen) or two letters, one upper case, one lower case (e.g., “He” represents helium. This symbol had to be given two letters; there are more than 26 elements and H was already taken). 2. The net charge, representing the algebraic sum of the charges of the protons and electrons in the chemical species; this is placed in the upper right hand corner; He2+ represents a helium ion with no electrons because the algebraic sum of the nuclear charge (2+) and the charge from electrons (0) equals 2+ [(2+) + (0) = 2+]. |
Symbols for Elements |
| 3. The atomic number (Z), the number of protons in the nucleus is
placed in the lower left hand corner; this is redundant information,
because if you know the identity of the element, you also can find its
atomic number Z from the periodic table.
4. The mass number (M), the total number of protons and neutrons in the nucleus, is placed in the upper left hand corner. |
Atomic number
Mass number |
As an example of this naming system, we examine three different kinds of
11H+ |
Isotopes |
A deuteron is a nucleus containing one proton combined with one neutron. The atomic number of the deuteron is the same as that of the proton (Z = 1) because the deuterium nucleus is still chemically identified as hydrogen. However, the deuteron has a mass number equal to 2 (M = 1 proton mass + 1 neutron mass = 2). Again, the deuteron nucleus contains no electrons and therefore has a positive charge. A deuteron is therefore represented as 12H+. The deuterium atom, containing one electron and one deuteron nucleus, would be neutral ( 12H ), i.e., no + charge in the upper right hand corner of the symbol, because the single negative electron neutralizes the single positive charge of the deuteron nucleus to yield a neutral atom. |
Proton vs. deuteron |
When discussing hydrogen isotopes, the symbolism H-1, H-2, and H-3 is often used to describe hydrogen isotopes containing one proton in combination with 0, 1(deuterium - D), and 2(tritium - T) neutrons, respectively. Physical properties of chemical species containing different hydrogen isotopes differ. For example, heavy water (D2O) containing all deuterium atoms has different properties from light water (H2O). D2O has a density (see below for a definition of density) that is larger than H2O and thus has been given the name heavy water. Heavy water is used in some types of nuclear reactors because of its heavy hydrogen isotope. A neutron, represented in nuclear equations by the symbol 01n , has a mass number = 1, contains no protons, and is electrically neutral. |
Radioactive Nucleii |
| Atomic mass is the mass of a particular atom relative to the standard atomic mass of the carbon-12 isotope of exactly 12. Note that no atomic mass is an absolute mass, but instead allows comparison among various atoms of their relative masses. Atomic mass is inappropriately sometimes called “atomic weight.” One atomic mass unit (amu) is one-twelfth the mass of a carbon-12 atom. Using this reference standard mass, the H-1 isotope is 1.00728 amu and the oxygen isotope O-16 is 16.000 amu. Elements found in nature consist of a mixture of isotopes. For example, hydrogen contained in water consists mostly of H-1 with small amounts of the heavier isotopes H-2 and H-3. Thus, the atomic masses of natural substances include these mixtures of naturally occurring isotopes. Periodic tables lists the average of the atomic masses of all the naturally–occurring isotopes of an element. The average atomic mass of hydrogen is listed as 1.00794 amu because of the relatively small amounts of hydrogen isotopes heavier than H-1 present in nature. |
Atomic Mass |
Example 1-3 Atomic numbers and mass numbers What are the mass number, the atomic number, and the number of neutrons in the
tritium nucleus The mass number is 3 for tritium. The atomic number is 1, because there is only one proton. To obtain the number of neutrons, we subtract the number of protons (1) from the mass number (3) and find that there are two (2) neutrons. To check our answer, we add the number of protons (1) and neutrons (2) and the sum (3) should be equal to the mass number listed on the upper left hand corner of the isotope. |
|
Example 1-4 Atomic numbers and mass numbers What are the mass number, atomic number, and number of neutrons in He-3? (Remember He stands for a helium.). Locate helium in the periodic table. |
|
Nuclear and chemical reactionsChemical reactions and nuclear reactions are events in which a substance is or substances are transformed into one or more new substances over a period of time that can range from fractions of a second to years. |
|
In a chemical reaction, all atoms retain their identities during the reaction, but are transformed into new arrangements of the same atoms in space forming new substances called products. The substances that react with each other to form products are called reactants. An example of a chemical reaction between two hypothetical molecules, designated A and B, is shown on the next page. During a collision between A and B, a chemical reaction takes place and the atoms in A and B are rearranged into two new molecules (C and D) if the collision is sufficiently energetic.
|
Chemical
reaction Products & reactants |
| In a nuclear reaction, one or more elements can be transformed into new elements through a collision of nuclei with each other or by the transformation of one radioactive element into another by the emission of a particle from its nucleus. In both of these types of reactions, energy can be absorbed or released. Many nuclear reactions of interest give off large quantities of energy compared with chemical reactions, for example in nuclear reactors or nuclear weapons. | Nuclear reaction |
Both chemical and nuclear reactions can be represented as equations. In a mathematical equation, e.g., (3 + 2) = 5, the equals sign represents an identity. In a chemical or nuclear equation, the equals sign is replaced by an arrow generally pointing from left to right. To the left of the arrow are the chemicals present before the reaction, the reactants, and to the right of the arrow are the chemicals resulting from the chemical or nuclear reaction, the products. Thus, in a hypothetical chemical or nuclear reaction in which reactants A and B react to form the products C and D, the reaction is written as reaction (1-1): A + B --> C + D (1-1)
All atoms involved in chemical reactions retain their identity. That is, in a chemical equation a hydrogen atom may be a part of the chemical A and remains a hydrogen atom when it winds up in the chemical D. |
Equation |
| For nuclear reactions, we generally need to write equations with more isotopic information than with chemical reactions because elements may change their chemical identity during the reaction. In the example below, we utilize isotopic symbols to represent a specific nuclear reaction, one of the simplest nuclear reactions that is thought to have occurred immediately following the Big Bang. |
Nuclear Reactions |
|
An Example of a Nuclear Reaction in the Big Bang Plasma It is suggested in Chapter 2 that an important nuclear reaction in the Big Bang
plasma was the interaction of a neutron and a proton as reactants to form a deuteron
and a gamma ray as products. A gamma ray (γ) is an energetic radiation with zero
mass and zero charge originating in many nuclear reactions and is given off during the
radioactive decay of many unstable nuclei. One can think of a gamma ray as a highly
energetic x-ray. Gamma rays carry away large quantities of excess energy and are
absorbed in surrounding matter, resulting in the generation of heat and chemical
damage to that matter. The above word description of this nuclear reaction is
represented in diagram form in Fig. 1-2: |
Gamma (γ) ray |
|
|
Typical nuclear reaction |
|
The same nuclear reaction depicted in Fig. 1-2 is represented below in equation form in (1-2): 11H+ + 10n → 21 H+ + gamma ray (γ) (1-2) In all nuclear reactions, the sum of the mass numbers of the reactants must equal the sum of the mass numbers of the products (the numbers found in the upper left hand superscripts of reactants and products). This same relationship applies to the sums of atomic numbers of reactants and products (found in the lower left subscripts of reactants and products). Furthermore, the algebraic sum of the electrical charges of the reactants must equal the (algebraic) sum of the electrical charges of the products. Check to see that all of these relationships are valid in equation (1-2). The gamma ray has no mass or charge. |
|
Chemical Equation |
|
The process in Fig. 1-3 also can be represented by the following chemical equation: H + energy → H* (excess energy) → H+ + e– (1-3) |
Ionization |
Advanced Topic: Reaction Kinetics
Volume and densityVolume is the measure of the bounded space occupied by an object. Volume can be calculated from the dimensions of an object and can be measured in units such as quarts, gallons, or liters. One liter (1 L) is approximately the same volume as one quart. One milliliter (1 mL) is one thousandth of a liter and is equal to the volume of a cube one centimeter on an edge
|
Volume |
|
|
||
Exercise 1-6 Determination of Density There are 314.4 grams contained in a 40.0 cubic centimeter block of pure iron. What is the density of iron? d = m/v = 314.4 grams/ 40.0 cm3 = 7.86 g/cm3 The above equation reads: density is equal to the mass per unit volume; for example, when 314.4 grams of a substance are contained in 40.0 cm3, this means there are 7.86 g of that substance in every cubic centimeter of that substance. This density is obtained by dividing 314.4 grams by 40.0 cm3. Note that the units grams and cm3 belong to the numbers 314.4 and 40.0. They are both experimental quantities, not pure numbers. Exercise 1-7 Density of water at room temperature If the density of water is 0.998 g/cm3 at 20°C, how much volume will one gram of water occupy? (Hint: if d = m/v, then d v = m and therefore v = m/d. Remember to put units with each number you write and make sure units cancel properly in any calculation you perform.) |
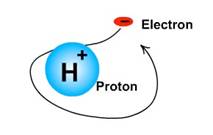


 The proton is a hydrogen atom nucleus with both an atomic number and a mass number of one and therefore has a single positive charge. The proton is one of three isotopes of hydrogen. According to the rules outlined above, the appropriate symbol for the proton is represented as:
The proton is a hydrogen atom nucleus with both an atomic number and a mass number of one and therefore has a single positive charge. The proton is one of three isotopes of hydrogen. According to the rules outlined above, the appropriate symbol for the proton is represented as:



